Aerosol administration of drug therapies is a well-established and increasingly important delivery method for patients with acute or chronic respiratory conditions, especially for those receiving critical care. Aerosol therapies are now given to one-quarter of critically ill patients and one-fifth of ventilated patients, and they are widely used in chronic respiratory conditions, particularly chronic obstructive pulmonary disease (COPD) and asthma.1–5 Additionally, nebulisers are widely used for patients with cystic fibrosis (CF) in both hospital and home settings.6,7
A number of factors affect aerosol delivery including lung anatomy in relation to airway geometry and branching and narrowing of the airways.8 Mucociliary clearance mechanisms and humidity can also impede the inhalation of drugs.9 Additionally, factors associated with the type of ventilation, including tidal volume, inspiratory time and duty cycle, inspiratory flow, waveform and bias flow can affect aerosol deposition.10 The lung offers an attractive site for delivery as it has a large surface area and thin epithelium which permits rapid drug absorption. Drug delivery through the lung is also a non-invasive route for drug administration.9 When levels of drug absorption into the circulation are low, administration through inhalation is associated with fewer side effects than systemic administration. If, however, there is large and rapid absorption there can be more side effects. This painless delivery can provide a more rapid onset of action, have a higher therapeutic index and achieve higher pulmonary tissue concentrations than many systemically administered drugs.8 This is particularly important in the treatment of pulmonary infections.
Aerosols of drugs introduced to the breathing circuit need to be created efficiently with minimal drug wastage. The size of aerosol droplet should be consistent and small enough to facilitate airway penetration so that a high proportion of the dose is deposited into lung tissues.9,11,12 Jet nebulisers (JNs) have been used to administer drugs for around150 years and are widely accepted. Aerosol droplet size for JNs, however, can be inconsistent and often there is incomplete nebulisation of the dose in the nebuliser reservoir. This results in variability of the actual dose delivered to the airways and lungs, which is a key factor affecting therapeutic response and is important when administering drugs such as antibiotics; for example, an imaging study reported that only 3% of the dose placed in the nebuliser actually reached the ventilated patient’s lungs in the specific conditions studied.13
The increasing prevalence of COPD and asthma is creating substantial healthcare burdens and is diminishing quality-of-life worldwide.14–17 These diseases impose high treatment costs where escalation of hospital care is required and there are consequent economic effects resulting from disability and incapacity.17–22 The World Health Organisation (WHO) estimates that 65 million people have COPD and believe it to be the current fifth leading cause of death, expecting it to rise to the third leading cause by 2030.23 Asthma exerts a significant burden in all territories in all age groups; it is estimated to affect between 235 and 334 million people worldwide and this prevalence is rising.24,25 Although the prevalence of CF is much lower, the associated lung disease requires chronic treatment which places a serious burden on patients, carers and healthcare systems. Treatments for COPD, asthma and CF during acute disease exacerbations are therefore of critical importance.
Efficient nebulisers and inhalers have the potential to improve cost effectiveness of treatments through shorter treatments and reduced drug wastage.26–29 Newer high-performance aerosol delivery such as vibrating mesh nebulisers (VMNs) are a vital means of rapidly administering medications such as bronchodilators, steroids and antibiotics. These maximise drug delivery with low residual volumes and some early data suggest that they may improve clinical outcomes more than other, less efficient aerosol generators.30,31 Early retrospective data support this and also suggest that efficient nebulisers and inhalers may reduce hospital admission rates and reduce the drug dosage needed for patients requiring bronchodilators in emergency departments. This results in improved patient throughput in busy acute care settings. The use of such treatments has the potential to rapidly improve a patient’s condition and may improve long-term outcomes.5,12,32,33
This review examines the evidence supporting the use of VMN devices and their current and potential roles in delivering drug therapy to patients with respiratory disease.
The vibrating mesh nebuliser
The VMN consists of recently developed aerosol technology that improves the delivery of aerosolised drugs to the lungs. The device has a very different design to conventional jet nebulisers. The key component is a central aperture plate that is perforated with precisely formed holes. A piezo ring vibrates the aperture plate which acts as a micro-pump drawing liquid through the holes to generate consistently sized fine particles of 1–5 µm diameter.12,34 This size of particle is advantageous because particles of 5–10 µm diameter will not penetrate beyond the larger lung airways.35
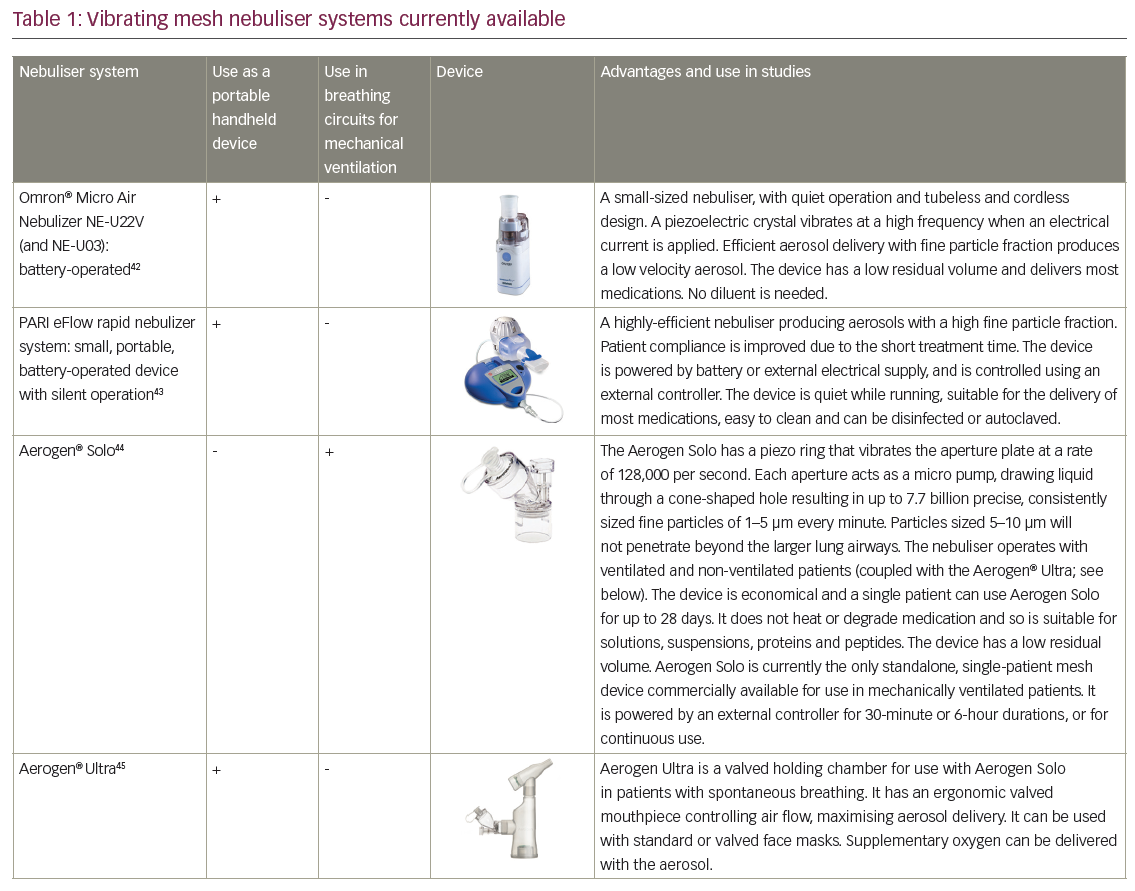
VMNs produce a low velocity aerosol which minimises ‘rainout’ (condensation of drug-containing solutions) in the circuit and upper airways thereby optimising drug deposition. They generate no heat and so maintain drug integrity.34,36,37 Several different VMN systems are available.12,34 One example is the Aerogen® Solo (Aerogen, Dangan, Galway, Ireland), which can be used during mechanical ventilation (MV), non-invasive ventilation (NIV) and high-flow oxygen nasal cannula ventilation (HFNC); its utility has been demonstrated in various clinical studies.30,31,38–41 Other VMN devices include the NEBU-TEC® M-neb®(NEBU-TEC International med. Produkte Eike Kern GmbH, Elsenfeld, Germany), which is also used in MV but there is currently little information supporting its clinical use. VMN technology is also successfully used to generate aerosols in handheld nebuliser devices such as the PARI eFlow (PARI Medical Limited, West Byfleet, Surrey, UK) and the Aerogen® Ultra (Aerogen, Dangan, Galway, Ireland) (Table 1).42–45
VMNs have a number of further advantages over JNs especially when used with MV systems. Some VMNs can remain in the circuit for up to 28 days with no influence on the gas flow when delivering nebulised drugs.1,46,47 VMNs do not alter air flows or pressures in the circuit and require less frequent intervention from medical staff.1,47 JNs require switching in and out of an MV circuit every 24 hours, which can be time consuming. Such processes can also be hazardous since there is a risk of lung derecruitment in sicker patients, after which re-establishing airflows can take time and opening the circuit can create opportunities for introducing infection leading to pneumonia.48 Reduced need for intervention from nursing staff and reduced infection risk have the potential to reduce costs of treating patients on MV.49 Delivery of some viscous drugs, suspensions and solutions prone to crystallisation on drying can sometimes clog the pores of VMNs. This can usually be avoided by nebulising a few drops of normal saline at the end of the nebulisation period to clear the pores. There is also some variability in VMN output and nebulisation times, but this has limited practical impact in the hospital setting. In spontaneous breathers there is a requirement to disassemble the VMN from its valved adapter to allow it to air dry between uses. Although more efficient, VMNs are generally more expensive than JNs, but in the intensive care unit (ICU) setting a VMN can be used for up to 28 days in a single patient.
Another type of aerosol generator is the ultrasonic nebuliser (UN). These were first developed in the 1960s and are also used to treat airway diseases. These nebulisers have a piezoelectric crystal that vibrates at high frequencies (1–3 MHz) in a liquid to form an aerosol.50 These devices are small, easy to use, require no air compressor, have quiet operation and, like VMNs, are more efficient aerosol generators than the JNs. UNs are mostly used for the administration of hypertonic saline but can also be used for the delivery of inhaled medications.51 The main disadvantage of UNs is that heat is generated in the process of producing aerosol; this can break down complex proteins in some inhaled medications. In addition, UNs are not recommended for administration of suspensions such as Pulmicort® (budesonide).1 They are therefore unsuitable for administration of suspensions and proteins. They are also unable to create aerosols from viscous solutions and have a large residual volume. Both UNs and VMNs have controllers that drive them, but the UN’s controller is bulkier and heavier. The UN has a reservoir that is positioned below the ventilator circuit whereby contaminated fluids in the circuit can more readily enter the nebulizer.1 Some comparative in vitro studies have shown that UNs have a fast delivery but can have poorer efficiency and produce larger particles than VMNs.29,50,52,53
Vibrating mesh nebulisers used with mechanical ventilation
MV is used for the most seriously ill and critical patients, but more recently there has been a general trend towards less invasive means of ventilatory support for many such patients.54–56 Aerosol therapy is frequently introduced into mechanical breathing circuits and is used by over 95% of intensivists, mostly for bronchodilator, antibiotic and steroid administration,51,57–59 and less frequently for anticoagulants, diuretics, mucoactive agents, prostacyclins and surfactants.46
One recent in vitro study compared the performance of four different nebulisers; the SideStream® Disposable JN (Philips Healthcare Limited, Guildford, Surrey, UK), the Multisonic® InfraControl UN (Flores medical GmbH, Probstzella, Germany), the Aerogen® Pro VMN and the Aerogen® Solo VMN (Aerogen, Dangan, Galway, Ireland) to deliver salbutamol in a model MV system.52 The UN was the fastest system to nebulise the dose; the JN was the slowest. Solution temperatures increased with the JN and UN, but decreased with the VMNs. Osmolarity increased with the JN and UN, but was stable with the VMNs. Salbutamol delivery was 2.3- and 1.6-fold higher with the VMNs and UN, respectively, compared with the JN. In addition, particle size was significantly greater with the UN (mean mass distribution: JN: 5.00 ± 0.36 µm, UN: 5.80 ± 0.07 µm, VMNs: 5.14 ± 0.54 µm (Aerogen Pro) and 4.60 ± 0.54 µm (Aerogen Solo); p<0.01 for UN versus VMN (Aerogen Pro). Overall, the VMNs and the UN were more efficient than the JN, but the VMNs did not heat the drug and produced a substantially higher respirable fraction.
Several bench and imaging studies have demonstrated suitability of VMN technology for use in MV circuits and improved drug delivery of aerosolised solutions.52,60–63 These investigations generally showed that aerosol devices are more efficient when placed at the ventilator or humidifier and less efficient at or near the Y-piece when bias flow was present (Figure 1).61

Berlinski and Willis showed that a VMN delivers ninefold more aerosol dose compared with a JN during simulated mechanical ventilation.61 A further bench study using both paediatric and adult breathing model systems showed that nebuliser placement prior to the humidifier increased drug delivery to a greater extent with a VMN than with a JN and that higher bias flow reduced drug delivery.64 In both model systems drug delivery with the VMN was two- to fourfold greater than with the JN at all positions (p<0.05).
A randomised, controlled study compared the in vivo impact of two ventilation modalities – pressure support versus volume-controlled ventilation – on lung dose of a radiolabelled aerosol administered with a VMN during invasive mechanical ventilation and was measured using scintigraphy.39 The study enrolled postoperative neurosurgery patients who were mechanically ventilated with healthy lung function and found that controlling the ventilatory pattern in volume control mode was associated with higher lung deposition compared with spontaneous ventilation in pressure support mode (15.1% versus 10.5%; p<0.05) (Figure 2). These levels of deposition are considerably higher than the deposition of 3.0% observed with standard JNs.13 Lung dose and the site of deposition were highly variable among patients with both ventilation modes.

The performance of VMNs have also been demonstrated in animal models. One example used a macaque model of a neonate and compared a JN (Misty-Neb [CareFusion, San Diego, CA, US]) with a VMN (Aerogen Pro [Aerogen, Dangan, Galway, Ireland]). This study used scintigraphy to demonstrate that 0.5% and 12.6% of 99mTc-diethylenetriamine pentaacetic acid (DTPA) was delivered to the lungs using the JN and VMN, resepectively.65 This and other studies indicated that the advantages of VMN are mainly driven through lower residual volumes.
Early clinical data suggest that the higher drug deposition associated with VMNs can improve pulmonary mechanics. One study included 25 children (aged 1–18 years) with respiratory failure (the majority had postoperative respiratory failure with pulmonary oedema) who were treated with salbutamol delivered by VMN.66 At baseline, functional residual capacity was only 53.0% of that predicted. After aerosolised salbutamol, functional residual capacity increased by 18.3% (p=0.008). The authors concluded that in infants and children, salbutamol aerosolised by VMN might favourably enhance pulmonary mechanics and thereby represent a novel strategy for lung recruitment in children with respiratory failure.
Several studies in the US have demonstrated substantial cost savings when switching from pressurised metered dose inhaler (pMDI) to VMN administration of drug treatments during MV. This effect is partly due to the relatively higher costs of pMDIs in the US.67 In these studies, switching from pMDIs to VMNs resulted in projected cost savings of approximately $150,000 to over $1.7 million per year depending on the numbers of hospitals involved. In some of these studies, the savings were made in parallel with improved patient and/or medical staff satisfaction over pMDI aerosol administration (Table 2).49,68–70 Little information on cost savings associated with VMN use with mechanical or other ventilator types in Europe is currently available.

Lung infections are a serious risk in patients with respiratory disease, particularly in those receiving MV, and they can be difficult to treat.7 VMNs in combination with MV have been successfully used to deliver antibiotic aerosol treatments directly to the lung in several studies. In one study of 165 patients with ventilator-acquired pneumonia (VAP), treatment with aerosolised colistin from a VMN (Aerogen Pro). achieved cure rates of 66% in patients with antibiotic-sensitive Pseudomonas aeruginosa or Acinetobacter baumannii infections and 67% with multidrug resistant infections of these bacteria.71 This efficacy was non-inferior to intravenous treatments with β-lactams, aminoglycosides or quinolones for treating VAP. A similar study that included 149 critically ill adults found that VMN-aerosolised colistin achieved a clinical cure rate of 67.1% in P. aeruginosa and A. baumannii infections compared with 72.0% receiving intravenous colistin (p=0.59).72 The aerosol-treated patients also had significantly lower incidence of acute renal failure (p=0.004), shorter time to bacterial eradication (p=0.023) and earlier weaning from the ventilator (gain of 5 ventilator-free days). In an international survey of aerosol therapies administered during mechanical ventilation,51 80% of 854 analysed responses had a positive opinion of nebulised colistin and 30% reported using nebulised antibiotics at least every other month.
Vibrating mesh nebulisers used with non-invasive ventilation
Non-invasive ventilation (NIV) is increasingly used as a less invasive option when MV can be avoided. NIV can be associated with lower morbidity than MV and reduces the risk of VAP.73–75 Some patients with severe COPD receive intermittent NIV in the homecare setting.76,77 A variety of bench studies have demonstrated the use of VMNs during NIV using simulated breathing patterns.78–81 One such NIV model compared terbutaline aerosol generated by VMN and a JN. The VMN produced aerosols that contained significantly more drug than the JN and this difference was more marked when the nebuliser was positioned before the leak port in the ventilation circuit (p<0.001 for VMN versus JN and p<0.001 for position comparison).78 Another bench study using a face model/respiration simulator showed that the proportion of drug(99mTc-salbutamol) inhaled via aerosol using a VMN was substantiallygreater than with a JN for three different NIV settings (VMN:14.3–15.4% versus JN: 3.6–7.2%; p=0.004–0.094).79 A comparison of a VMN, JN and pMDI used in an NIV model showed that the VMN was superior in terms of salbutamol delivery (p<0.001) and that placing each nebuliser before the leak port provided the best drugdelivery (p<0.001).81
An NIV in vitro study compared the performance of three VMNs (Aerogen Pro, Aerogen Solo, and NIVO® [Philips UK Ltd, Guildford, Surrey, UK]), one JN (Sidestream), and one UN (Servo Ultra Nebulizer 145 [Siemens-Elema AB, Solna, Sweden]) coupled with a single-limb bilevel ventilator in the delivery of amikacin.29 When the nebuliser was positioned before the exhalation port in the circuit, the VMN delivered the highest inhaled dose (p<0.001), the JN showed the highest expiratory wasted dose (p<0.001) and UN had the highest total lost dose (p<0.001). When the nebulisers were placed after the exhalation port, however, the VMN showed the highest expiratory wasted dose. The authors concluded that UNs are not recommended in this NIV application and that the VMNs were the most efficient provided they were placed before the exhalation port.
Clinical evidence supporting the use of VMNs with NIV in respiratory disease is, as yet, limited. In a crossover study, 10 healthy volunteers received 99mTc-DTPA aerosol treatment via VMN or JN connected to an NIV circuit using a facemask. Radioactive counts and scintigraphy images showed that healthy subjects received greater than threefold more radioactive drug during VMN treatment than during JN treatment.82 This evidence indicates that there is potential for VMN devices to be used in combination with NIV as successfully as with other ventilation approaches, but larger comparative trials are needed.
Vibrating mesh nebulisers used with high-flow oxygen through nasal cannulas
Therapy with high-flow oxygen through nasal cannulas may offer an alternative to MV and NIV in some patients. A number of different bench studies have demonstrated VMN aerosol medication delivery via HFNCs and explored the optimal position of the nebuliser in the circuit.11,83–86 A model HFNC system with a VMN placed on the patient side of a humidifier found that cannula output ranged from 8.45–26.90% of the loaded dose depending on the variable flow rates.11 The median particle sizes generated were 4.2 ± 0.4 µm for adult cannulas and3.8 ± 0.5 µm for paediatric cannulas. A study with a model paediatric breathing circuit showed that when using a VMN with a HFNC, decreasing the flow of oxygen or heliox (helium and oxygen) from 6 L/min to3 L/min increased salbutamol lung deposition delivery twofold or greater (p=0.028 and 0.002, respectively).83 At 6 L/min drug deposition was twofold or greater with heliox than with oxygen (p=0.01). A further in vitro study using three different HFNC systems found that a10 L/min air flow provided the best drug delivery.84 At 30 L/min and50 L/min the size of the cannula made a significant difference (p<0.001). A study modelling HFNC with a VMN or JN in adults showed similar findings in that more drug (salbutamol) was delivered to the lungs with an air flow of 30 L/min rather than 45 L/min or 60 L/min. This delivery was not affected by high inspiratory flows as would be seen during respiratory distress.85 This study also found that the most efficient position of the nebuliser was before the humidifier allowing greater drug delivery beyond the nose and pharynx.
Despite the improved efficiency of VMNs compared with JNs used in HFNC, the total proportion of drug nebulised with HFNC is lower than with other ventilator methods. A recent scintigraphy study included six healthy subjects and compared lung deposition using 99mTc-DTPA(4 mCi/4 ml) administered via a VMN (Aerogen Solo) or a JN (Opti-Mist Plus [ConvaTec Limited, Deeside, Flintshire, UK]) via an HFNC (Optiflow™ [Fisher & Paykel Healthcare Limited, Auckland, New Zealand]).38 In the specific conditions of the study, with a flow rate of 30 L/min, lung deposition was 3.6% of the nominal dose with the VMN compared with 1.0% for the JN. Both nebulisers were associated with substantial deposition in the single limb circuit, the humidification chamber and the nasal cannula (58.2% versus 19.2%; p<0.05) and in the upper respiratory tract, especially in the nasal cavity (17.6% versus 8.6%; p<0.05).
A further study of VMN used in a HFNC system in 23 healthy adult volunteers confirmed effective delivery of 99mTc-DTPA.87 Scintigraphy images showed the greatest deposition occurred at 10 L/min compared with 30 L/min or 50 L/min, and that there was a strong inverse correlation between lung deposition and air flow in both heated and unheated conditions (r2= -0.008 and -0.597, respectively). Overall, aerosol administration via HFNC was seen to be a viable option for delivering clinically relevant doses to the lung. It should be stressed that in the acute setting, physicians use more than 50 L/min or60 L/min to provide positive pressure and increase oxygen saturation levels to adult patients. In some cases, however, it may be necessary to decrease flow to deliver a meaningful dose of bronchodilator in severe bronchospasm; with VMNs the duration of nebulisation is shorter than with JNs keeping times of lower flow to a minimum.
In an interim analysis of an ongoing, randomised, crossover clinical study, 13 adult patients with respiratory failure received a nebulised bronchodilator via a VMN through HFNC, via a standard JN and facemask or just HFNC with sham nebulisation (no drug). HFNC was operated with a low airflow of 30 L/min.41 The absolute and relative changes in forced expiratory volume in one second (FEV1) were: forHFNC/VMN: 330 ml, +17%; for standard facemask/JN: 350 ml, +18% and for HFNC and sham nebulisation: 40 ml, +2%. This interim analysis suggests that at 30 L/min airflow, the HFNC/VMN treatment was non-inferior to standard facemask/JN treatment and that both were greatly superior to HFNC with sham treatment.
Additional evidence supporting the use of VMNs with HFNC was provided by a case series of five infants with asthma and bronchiolitis due to rhinovirus or enterovirus infection who were initially treated with bronchodilators via JN and standard facemask, and then switched to HFNC with VMN.88 Following treatment with HFNC and VMN the infants became markedly less agitated than with the JN and facemask, suggesting that HFNC with VMN was clinically better tolerated, possibly preventing them from escalating to more invasive respiratory support. Mean heart rate was also substantially higher at 187 beats per minute for HFNC/VMN compared with 138 beats per minute for facemask/JN administration. The authors believed that this reflected greater delivery of drug using HFNC/VMN.
Vibrating mesh nebulisers in spontaneously breathing patients
VMNs have been used to generate aerosolised drug delivery in many spontaneously breathing patients with respiratory difficulties.3,89,90 The use of VMNs in this indication is supported by a range of bench studies that have demonstrated their advantages over JNs in terms ofdrug deposition.
Hickin et al.28 compared the dose and rate of drug delivery of a VMN with a standard JN fitted to a standard face mask in a breathing model simulating a normal patient and one having a COPD exacerbation. The VMN delivered salbutamol at a significantly higher rate than the JN (257.9 µg/min versus 11.9 µg/min) in simulated healthy patients (p=0.008). In simulated COPD, these figures were 109.8 µg/min and 8.5 µg/min (p=0.005) meaning that nebulisation time was almost halved. Furthermore, the VMN delivered nearly eight times the dose of salbutamol to the carina compared with the JN; less than 1% of the original dose remained in the VMN reservoir (residual) compared with over 40% in the JN reservoir (p<0.001).
Ari et al. conducted performance comparisons of JNs and VMNs using different interfaces in simulated spontaneously breathing adults and children.90 Drug delivery was shown to be greater for the VMN than JN when using a mouthpiece (15.4% versus 7.7% of nominal dose) or a valved mask (15.2% versus 8.6%), but was similar when using an aerosol mask (7.5% versus 6.8%).
Another model study investigated the risk of exposing carers and bystanders to aerosols as a result of leakage from facemasks and filtered mouthpieces used in combination with JNs. Simulated patient models showed that this risk is lower with VMNs than other aerosol devices due to the fact they generate lower velocity aerosols.91 Such exposure risk is also decreased with a valved mask and mouthpiece.
In a scintigraphy study, spontaneously breathing healthy volunteer subjects (n=6) were treated with radio-aerosol (99mTc-DTPA) via a VMN (Aerogen Solo plus Aerogen Ultra) or a JN (NS, São Paulo, Brazil).92 The VMN achieved pulmonary deposition with four to six times greater efficiency compared with a JN (22.8% versus 4.5% of dose delivered to the lungs; p=0.004). The JN also had a much greater residual volume resulting in lower drug delivery to the subject (Figure 3A). Further evidence of VMN efficiency comes from an imaging study with a crossover design that included six healthy male subjects. Single-photon emission computed tomography (SPECT-CT) was used to determine lung penetration of 99mTc-DTPA that was administered via a VMN (Aerogen Ultra) or a JN (Opti-Mist Plus).93 Pulmonary aerosol deposition from SPECT-CT analysis was six times greater with the VMN compared with JN (34.1% versus 5.2%; p<0.001) (Figure 3B). However, aerosol penetration expressed as the three-dimensional normalised ratio of the outer and inner regions of the lungs was similar between the VMN and JN.

VMNs have also shown efficacy in the administration of bronchodilators to spontaneously breathing patients with COPD. In a pilot study,30 patients with an acute exacerbation of COPD were treated with asingle bronchodilator dose (2.5 mg salbutamol/0.5 mg ipratropium bromide) using a VMN (Aerogen Ultra) or a JN (Hudson Micro Mist [Teleflex Medical Europe Limited, Athlone, Ireland]).30 The VMN group demonstrated a significant improvement over JN in forced vital capacity (p<0.05) and a greater volume response to bronchodilators with clinically significant increases in inspiratory capacity (>10% of predicted) and a clinically significant reduction in residual volume (>300 ml). There was also a significant improvement in the Borg breathlessness score from baseline with the VMN, with a trend to significance between the two nebulisers (p=0.08). The authors concluded that since exacerbation recovery has been associated with increases in respirable lung volume, it is possible that greater bronchodilator delivery with VMNs may hasten exacerbation recovery, although this has yet to be explored.
Additional evidence showing the advantages of VMNs in spontaneously breathing patients came from a retrospective analysis of a large body of emergency department patient data (n=1,594), which compared the efficacy of VMN (n=879) (Aerogen Ultra) with JNs (n=715) (brand of JN not specified) in the administration of a bronchodilator (salbutamol)(Figure 4).31 Patients receiving JN treatment were 1.7 times more likely to be admitted to hospital than those receiving treatment with a VMN (p<0.001). Patients receiving VMN treatment had significant reductions in median length of emergency department stay (-13%) and were 1.3 times more likely to be discharged than those receiving JN treatment (p<0.001).31 The VMN group used less total drug (p<0.05) with a 75% reduction of maximum salbutamol dose administered (20 mg to 5 mg) (Figure 5).31


In patients with CF, pulmonary infection is a persistent risk and cause of significant morbidity and mortality.7 VMNs in handheld devices have been used in several pharmacokinetic studies to investigate antibiotic administration, particularly tobramycin, to treat P. aeruginosa and other Gram-negative bacterial infections in spontaneously breathing patients. One open-label, crossover study of 27 patients with CF and pulmonary P. aeruginosa infection investigated tobramycin(300 mg/4 ml) administrated via the PARI eFlow VMN nebuliser or the PARI LC Plus (a ‘breath-enhanced’ nebuliser for home use that uses a valve system to enhance delivery in a similar manner to the Aerogen Ultra). The VMNs produced similar pharmacokinetic profiles in plasma and sputum, but with shorter nebulisation times for the PARI eFlow device.94 A similar crossover study of 58 patients with CF and pulmonary P. aeruginosa infection compared 170 mg tobramycin/1.7 ml via an eFlow device with 300 mg tobramycin/5 ml via a PARI LC Plus device.95 The 170 mg tobramycin/1.7 ml treatment produced higher area under the curve (AUC) and maximum concentration (Cmax) values but a lower systemic burden and shorter inhalation times, making it a favourable option for patients with CF. In a further crossover study of 29 patients with CF and chronic P. aeruginosa infection, tobramycin inhalation solution was administered via a VMN (PARI eFlow) once daily for 8 weeks, then twice daily for 8 weeks.96 The AUC0–90min ratio at 8 weeks and mean FEV1 did not differ markedly between the treatments and no nephrotoxic or audiological side effects were noted. This indicated that both once- and twice-daily tobramycin inhalation solution aerosol dosing achieves effective drug levels and both are tolerable. Overall, these studies indicate that VMN-nebulised antibiotics have considerable potential for the routine administration of antibiotics in CF.
Breath-enhanced/actuated nebulisers (BANs) are important frequently used aerosol generators in the treatment of spontaneously breathing patients. In a recent spontaneously breathing model study, a BAN (AeroEclipse® [Monaghan Medical Corporation, Plattsburgh, NY, US]) was compared with a VMN (Aerogen Solo plus Ultra).97 The VMN delivered two- to threefold greater drug dose than the BAN, with lower residual volume left in the VMN. Clinical investigations of BANs include a randomised controlled study by Sabato et al.98 In 149 spontaneously breathing paediatric patients with asthma in the emergency department, bronchodilator administration was compared using either a small volume standard JN (SVN), a large volume JN (LVN) or a BAN (AeroEclipse®). Length of stay in the emergency department was not reduced; however, patients in the BAN group showed a greater improvement in clinical asthma score, respiratory rate and a significantly lower admission rate (38% versus 57%; p=0.03). In an editorial review of this article, Ari and Fink identified a few confounding factors between the groups (SVN versus LVN versus BAN).98,99 The authors concluded that the method of delivering the bronchodilator could have impacted on the outcomes of the study.
Discussion and future directions
The evidence discussed above indicates that in both in vitro systems and patient studies investigating various ventilation modalities, VMNs showed greater efficiency in delivering drugs to lung tissues than JNs, pMDIs or UNs. This has the potential to improve patient outcomes; however, few randomised controlled trials studying patient outcomes have been published to date.3,12,47,100–102 VMN administration is applicable to adult use but the efficiency and size of the aerosol particles also make it suitable for paediatric use where penetrating very small airways can be an issue.39,58,82,101,103 While much of the evidence justifying VMN aerosol administration comes from studies that use handheld or portable devices, there are indications that the devices have considerable potential for routine use in breathing circuits for patients in respiratory distress.
Compared to JNs, the key advantages of VMNs include: shorter treatment times; lower residual volumes (<0.1 ml for 3.0 ml dose) resulting in less drug wastage and higher amounts of drug being delivered to patients; and the possibility to nebulise small volumes of drug. VMNs can be used with most medications for inhalation. They do not heat or degrade medications so they are suitable for most solutions, suspensions, proteins and peptides.1,34 In mechanically ventilated patients, the extended time that VMNs can be left in place in the circuit reduces the need for manipulation, making it possible to refill medication without breaking the circuit. Ventilator parameters are therefore not disturbed and there is potentially lower risk of introducing infection.94,104 Clinical trials are required to confirm the patient outcomes related tothese factors.
The recent pilot study by Cushen et al.,30 suggested that utilising VMN to improve the delivery of bronchodilators to the airways of patients with acute exacerbations of COPD may improve lung volumes. However, this was only a single dose study and a multiple dose study would be needed to determine whether the improvements in lung volume and symptom score can result in shorter durations of acute exacerbations. This could be important in terms of shortening hospital admissions for patients with COPD and the consequent impact on healthcare costs, but also in potentially impacting the progressive lung damage caused by severe COPD exacerbations.
The recent retrospective analysis of 1,594 patients by Dunne et al.31 also suggested that in patients requiring nebulised bronchodilators it may be possible to reduce hospital admissions and length of stay in the emergency department. The latter might be due to more efficient drug delivery to the airways but could also be due to shorter treatment times. If these results can be confirmed in randomised, controlled clinical trials this would suggest substantial hospital savings from reduced hospital admissions and improved patient throughput in often overcrowded emergency departments. Several studies in the US49,68–70 have demonstrated notable savings in drug costs when using VMNs as opposed to MDIs in the intensive care setting but, as yet, there is little evidence available on cost savings from studies on other nebuliser types or from studies conducted in Europe or elsewhere where the costs of MDIs are much lower.
In addition to bronchodilators, VMNs are also used for the administration of a variety of other treatments. The nebulisation of antibiotics for difficult to treat respiratory infections such as VAP or community-acquired pneumonia,71,72,105 or chronic and acute pulmonary infections in CF,7,101 has increased substantially over recent years. This is driven by the possibility of achieving higher antibiotic concentrations in the infected tissue while keeping systemic blood levels low and potentially improving the therapeutic index. This is important especially when some of the antibiotics required to treat multidrug resistant infections have serious systemic toxicity, but also given the concerns around antibiotic resistance and the need to control the doses of these powerful drugs.7,106
There are many publications outlining the use of VMNs for delivery of heparin for pulmonary inflammation in ventilated patients,32 antifungals for invasive aspergillosis,107–109 furosemide for COPD exacerbations, surfactant for neonates, prostacyclins for pulmonary hypertension, bronchopulmonary dysplasia and breathlessness in lung cancer,33,110,111 and the administration of insulin to patients with diabetes.112–114 However, many of these drugs are not approved for inhalation or are not specifically approved for inhalation with a VMN and are off label. Further clinical studies are needed to better support the use of VMNs in this wider range of indications.
A notable development in VMN systems in recent years has been the appearance of the so-called ‘smart nebulisers’ such as the I-neb Adaptive Aerosol Delivery (AAD) System (Philips Healthcare Limited, Guildford, Surrey, UK).115–117 These systems have the potential to improve nebuliser efficiency by adapting delivery in response to the patient’s breathing pattern. Smart nebulisers also have internet connectivity providing feedback to the patient and healthcare professionals via a smart phone for monitoring adherence, patient condition and device performance. The high cost of the I-neb AAD System is a potential downside. It is only for use in spontaneously breathing patients and can’t be used during mechanical ventilation.116
Respiratory conditions including COPD, asthma and CF impose increasingly serious health burdens on worldwide populations and VMN technology has the potential to improve aerosol treatments for these diseases. Bench models and imaging data confirm the high efficiency of VMNs to deliver drug to the lungs and airways across all ventilation modalities. Early clinical studies, especially in spontaneous breathing patients, may suggest that increased bronchodilator delivery to the airways and lungs could improve clinical outcomes in terms of shortening treatment times and potentially reducing hospital admissions and duration of exacerbations, but much larger randomised studies are needed to confirm this. There are many ongoing antibiotic delivery studies which will report in the coming years. Delivering more drug to the airways and lungs, therefore, has the potential to more rapidly and cost-effectively resolve acute exacerbations of respiratory disease than is currently possible and may positively impact outcomes in an increasing population of patients worldwide.