Diffuse panbronchiolitis (DPB) is a rare inflammatory lung disease. It was first identified in 1969 and is well recognized in East Asian countries such as Japan, China, Taiwan, and Korea.1 ‘Diffuse’ and ‘pan’ words in the name indicate ‘presence of lesions through both the lungs,’ and inflammation in all layers of bronchioles.
At the time of its discovery, DPB had poor prognosis because of recurrent respiratory infections leading to respiratory failure. In the years following the initial description of DPB in Japan, cases were also identified in other parts of Asia including China and Taiwan, thus giving it recognition as a distinct clinical entity. Over the years, sporadic DPB cases have been reported worldwide in countries other than Japan, China, Taiwan, and Korea (hereafter referred to as rest of the world [ROW]). This stimulated us to undertake a comprehensive search for available case studies on DPB. The aim of this study was to present the current clinical evidence captured as DPB case studies across the globe excluding East Asian countries.
A total of 657 studies regarding DPB were retrieved from PubMed in a systematic manner (Figure 1). Forty-eight case studies published in ROW and indexed in PubMed were included in the final review.
As indicated in Figure 2, the maximum number of DPB cases among ROW countries were published from the US (18.75%, n=9),2–10 jointly followed by Australia,11–15 France,16–20 and Italy21–25 (each 10.41%, n=5), respectively. There were three DPB cases published each from India26–28 and Turkey,29–31 and two case studies each from Belgium,32,33 Brazil,34,35 Germany,36,37 and Thailand.38,39 One case report each was reported from Canada,40 Ireland,41 Malaysia,42 Netherlands,43 New Zealand,44 Norway,45 Portugal,46 Singapore,47 Spain,48 and the UK.4
It is interesting to note that a majority (50%, n=24) of DPB studies were from four developed countries. Twelve of 48 studies (25%) reported DPB in patients of East Asian origin (either they immigrated to other countries or were born to East Asian parents). Patients were reported either by race (Caucasian,13,40 Hispanic, African-American4,9) or ethnicity (American citizens, White patients, Oriental [Asian] immigrant,3 Cambodian man, White man in Turkey,29 Turkish girl,30 Italian man,21,22 man of Indian ethnicity,44 Scandinavian patient,45 non-Asian Brazilian citizen,34 and Caucasian man of Canadian origin40). None of these patients with DPB had ever travelled outside of their country until DPB was diagnosed, except for one Hispanic patient with an extensive travel history to Far Eastern countries including Japan.5 Though most of the cases reported adult patients with DPB, two studies presented DPB cases in children. One case presented a 10-year-old child of Korean birth,10 and the other reported a 12-year-old Turkish girl with DPB. One study reported DPB recurrence in an African-American man after bilateral sequential lung transplantation.4

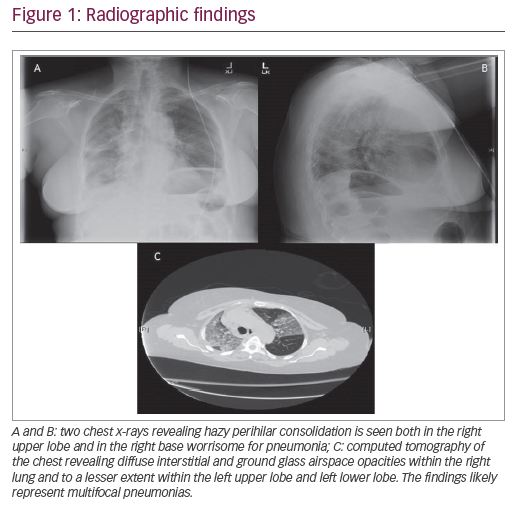
In the past, some publications had attributed lack of clinical awareness and diagnostic familiarity in ROW countries with under-diagnosis and failure of treatment of DPB.8 It was demonstrated that East Asians have more chance to possess the human leukocyte antigen (HLA) serotype—Bw54, which was demonstrated to be associated with DPB.50 In the present study, HLA-Bw54 serotype testing was carried out in 8 cases (16.7%), whereas high-resolution computed tomography (HRCT) was reported in 21 cases (43.8%). Other methods such as chest radiography (X-ray) or computed tomography (CT) were popularly utilized in different studies for clinical decision-making. Apart from these, 25 of 48 studies (52.1%) relied on histopathological investigations (surgical, bronchoscopy, open lung or trans-bronchial biopsy etc.) to confirm the diagnosis of DPB. The fact that there were a total of 13 DPB cases from six countries nearby to East Asia (Australia, 5 studies; New Zealand, 1; India, 3; Thailand, 2; Singapore, 1; and Malaysia, 1 study) raises concerns.
Since DPB treatment and prognosis are different from other obstructive pulmonary diseases, the chances of misdiagnosis are high. Early diagnosis, identification and early initiation of low-dose macrolide therapy can lead to improved survival. Patients with DPB described from ROW countries may also increase if this disease is taken into consideration in differential diagnosis of sinopulmonary diseases. Differential diagnoses of DPB include chronic bronchitis, bronchiectasis, primary ciliary dyskinesia, cystic fibrosis, hypogammaglobulinaemia, and bronchiolitis.51 Diagnostic criteria proposed in 1998 by a working group of the Ministry of Health and Welfare of Japan52 include: (a) persistent cough, sputum, and exertional dyspnea; (b) history of chronic paranasal sinusitis; (c) bilateral diffuse small nodular shadows on a plain chest X-ray (CXR) film or centrilobular micronodules on chest computed tomography (CT) images; (d) coarse crackles; (e) ratio of forced expiratory volume (FEV1) and forced vital capacity (FVC) <70%, arterial oxygen tension (PaO2) <80 mmHg; and (f) titer of cold hemagglutinin ≥64. Established DPB cases should fulfill criteria a, b, and c, along with at least two of criteria d, e, and f.

Multiple factors might be involved in the increased number of DPB cases from the ROW. Though nothing is known about the etiology of DPB, an increasing number of articles mentioning the importance of correct DPB diagnosis cannot be ignored. It is evident that lung biopsy remained the most reliable technique for confirmatory diagnosis of DPB (>50% cases) in ROW countries where the disease is rare and clinicians are not familiar with it. Additionally, HRCT is now preferred over CT scan and chest radiographs these days as it provides non-overlapping patterns which are highly suggestive of the diagnosis, when compared with CT. Also, being a non-invasive method to assess treatment response without the need for repeated histological evaluation, HRCT might help to retain patients with DPB until the end of treatment. Since macrolide levels in the airways of patients with DBP are well below the minimal level to exhibit antibacterial effects, anti-inflammatory and immuno-modulatory effects can be attributed to a beneficial effect of low-dose, long-term macrolide therapy.53
Established diagnostic criteria and treatment guidelines are well known in East Asian countries where the disease incidence is higher. But, as is evident from case studies in this article, the emerging global footprint of this disease raises concerns. There is a need to make clinicians aware and familiar with DPB, and to recognize it as global sinobronchial disease by discussing its diagnostic features to help pulmonologists able to recognize and treat this disease without delay. The observation that the majority of DPB case studies were identified in developed countries (US, Italy, France, and Australia [Figure 2]) can be attributed to increasing awareness amongst pulmonologists.
To conclude, the effects of DPB under-reporting due to lack of awareness and recognition leading to misdiagnosis and delayed (or lack of) treatment, cannot be ignored. Though sporadic cases from other geographical regions have been reported in past, the chances of DPB being misdiagnosed cannot be ignored. Timely diagnosis and intervention initiation can be life saving for many potential patients who might have been misdiagnosed.